Efficiency of Three Aquatic Macrophytes in Mitigating Nutrient Run-off
International Journal of Ecology and Environmental Sciences 31(1): 1-7, 2005
© INTERNATIONAL SCIENTIFIC PUBLICATIONS, NEW DELHI
Efficiency of Three Aquatic Macrophytes in Mitigating Nutrient Run-off
E. DEAVER1, M.T. MOORE2*, C.M. COOPER2 AND S.S. KNIGHT2
2USDA-ARS National Sedimentation Laboratory, P.O. Box 1157, Oxford, Mississippi 38655, USA
*(Corresponding Author; E-mail: mtmoore@msa-oxford.ars.usda.gov)
Ditches lining agricultural fields are used to convey runoff water and are typically mowed to reduce vegetative growth. If agricultural ditches are viewed as a wetland, ditch vegetation might be utilized to remove excess nutrients in runoff water. It was hypothesized that particular species of ditch vegetation would be more effective at removing nutrients in runoff water. Replicate 379 L Rubbermaid® troughs (132 cm ×70 cm × 66 cm) were planted with individual species of soft rush (Juncus effusus), yellow primrose (Ludwigia peploides) and cutgrass (Leersia oryzoides), common wetland macrophytes found in Mississippi agricultural drainage ditches. Nutrient-enriched water (target concentrations = 5 mg L 1 nitrate, 5 mg L 1 orthophosphate, and 5 mg L 1 ammonia) was pumped in at a 4 h hydraulic retention time at one end of the tub and discharged at the far end. Water samples were collected from discharge at 1 h intervals for 9 h and analysed for nutrient concentrations. Nutrient removal rates were compared for all plant treatments and unplanted sediment-water controls. Results indicated that no single species was most effective at removing both nitrogen species and orthophosphate, although all three plant treatments lowered nutrient concentrations in water relative to unplanted controls. Ammonia and nitrate concentrations in water were most decreased in Ludwigia peploides tubs (83±3% and 40±8%, respectively) and total orthophosphate was decreased most by Leersia oryzoides (29±7%). Leersia oryzoides was least effective at removing nitrate and ammonia concentrations. By determining specific plant retention of various nutrients, improved planning can be accomplished for best management practices and remediation techniques such as constructed wetlands or vegetated agricultural drainage ditches.
Key Words: Nitrate, Ammonia, Orthophosphate, Phytoremediation.
Eutrophication caused by excess nutrients is a pre- dominant water quality issue for many surface waters in the United States (US EPA 2000). Nutrients are the fourth largest impairment of water bodies in the US behind pathogens, metals and sediment (siltation). Slightly over 5000 (10%) of reported impairments are attributed to excessive nutrients (US EPA 2004). Non- point source runoff from agricultural fields may contain high levels of nutrients, particularly nitrogen (N) and phosphorus (P), and can be a major contributor to eutrophication of adjacent waters (Carpenter et al. 1998).
Within the last decade, much attention has been given to the hypoxic zone forming annually in the Gulf of Mexico. Many believe this zone is a result of growing nutrient concentrations, specifically nitrate (NO3), being transported down the Mississippi River into the Gulf. From 1980 to 1996, 61% of the mean annual total N flux into the Gulf was in the form of NO3, while 2% was as ammonium (NH4) (Goolsby et al. 2000). Donner (2003) suggested that since 1950, intensive cropping of corn, soybeans and wheat has impacted US land cover and water quality. On average, approxi- mately 35% of N in the Mississippi River dis-charged into the Gulf originated in the corn belt of Iowa and Illinois (Goolsby et al. 2000). Subsequent changes in nutrient ratios in the Mississippi River since that time could potentially be attributed to increased fertilizer usage.
Constructed wetlands have been shown to be effective in removing nutrients from runoff water (Hammer 1989, Moshiri 1993, Kadlec and Knight 1996), but most farmers cannot afford to take land out of production for use as a constructed wetland. The majority of agricultural lands in the lower Mississippi Valley and Gulf Coast areas have drainage ditches lining the fields to channel excess surface water flow away from crops (Bengston et al. 1995). These ditches are the link between farmland and larger bodies of water such as streams and rivers. Many of these ditches have standing water, or very wet soils and support growth of hydrophytic vegetation. If agricultural ditches are viewed as a type of wetland, they might be used to remove excess nutrients by encouraging growth of hydrophytic vegetation that will rapidly sorb nutrients. Previous studies by Moore et al. (2001) and Cooper et al. (2004) have indicated the value of vegetated agricultural drainage ditches in the Mississippi Delta for mitigation of pesticide-associated storm runoff. The present study involved the use of mesocosms with monocultures of three common emergent ditch macrophytes, soft rush (Juncus effusus), yellow primrose (Ludwigia peploides) and cutgrass (Leersia oryzoides). This study’s objective was to determine which of the three species of macrophytes, if any, was most effective at removing NO3, ammonia (NH3) and total orthophosphate (PO4) concentrations from nutrient enriched flowing water.
Experiments were conducted using eleven, 379 L Rubbermaid® troughs (132 cm × 70 cm × 66 cm) as mesocosms. Mesocosms were filled with 21.6 cm of sand in the bottom, covered with 16.5 cm of silt/clay and filled with 24.1 cm of pond water. Each mesocosm was planted with a single species of macrophyte and maintained for one year prior to initiation of the experiments. Three species of rooted, emergent-leaved macrophytes were planted: soft rush (Juncus effusus), yellow primrose (Ludwigia peploides) and cutgrass (Leersia oryzoides). Plants were collected from unused ponds located at the University of Mississippi Field Station, Bay Springs, Mississippi. Three replicate mesocosms were planted with each species. Two unvegetated control mesocosms contained only sediment and water. All mesocosms were randomly arranged.
Pond water (NO3, PO4 and NH3 concentrations below detection of 0.001 mg L 1) was pumped through the mesocosms for two days prior to the start of each study. For each experiment, nutrient enriched pond was prepared in reservoirs and pumped into individual mesocosms at the water surface. Nutrient stock was prepared using laboratory grade sodium nitrate, ammonium sulfate and potassium phosphate dibasic as sources for N and P species typically encountered in agricultural runoff. Water flowed through each meso- cosm and exited at the surface through a discharge hose at the opposite end of the mesocosm. FMI® metering pumps were used to deliver nutrient enriched water at a rate calculated to generate a 4 h hydraulic retention time (HRT). Water samples were collected from the discharge hose of each mesocosm at 1 h intervals for 9 h and analyzed for NO3, PO4 and NH3 according to Standard Methods (APHA 1998). Experiments were conducted three times; one per week in successive weeks of July 2002. Water quality (dissolved oxygen, temperature, pH, conductivity) parameters were measured in each mesocosm one week before the first experiment and again one day before the initiation of each experiment using YSI-85 meters. Plant density in each mesocosm was quantified by measuring area coverage and mean shoots m 2.
Statistical analyses utilized F-tests and t-tests for normally distributed parametric data (Ambrose and Ambrose 1995). Analyses were conducted with an alpha level of 0.5. Sample means for both percent nutrient removal and nutrient concentration were examined.
Leersia oryzoides had the greatest mean coverage (99%) and a mean of 820 shoots m 2 (Table 1). Ludwigia peploides had 97% coverage, with a mean of 312 shoots m 2. Two of the Juncus effusus mesocosms had less than 50% coverage and had a mean value of 2285 shoots m 2 (Table 1). It is expected that the amount of nutrients removed may be proportional to the total biomass of plant material in the canopy; therefore, nutrient concentrations were adjusted based on 100% coverage for all three plant species, plotted and reevaluated (Tables 3-5; Figures 1-3).
Water quality measurements indicated similar background conditions in all treatment and control mesocosms (Table 2). Analysis of nutrient concen- trations from the discharge of each mesocosm indicated similar trends in nutrient removal for all three trials of the experiment. All mesocosms exhibited increasing concentrations as nutrient enriched water was pumped water (5 mg L 1 NO , 5 mg L 1 PO4 and 5 mg L 1 NH) into the troughs. Concentrations typically began to decline after 6 h, as incoming nutrient concentrations were reduced. Control mesocosms had the highest nutrient concentration compared to all vegetated meso- cosms for the first 6 h and all three plant treatments lowered nutrient concentrations in the water relative to the unplanted controls. Results indicated that no single concentration of 0.32 mg L 1 NH (Table 3). Leersia plant species was most effective at removing NH3, NO3 and PO4.
Mean NH3 removal in Ludwigia peploides was statistically significant compared to both Juncus effusus and Leersia oryzoides. Ammonia concentrations in Ludwigia peploides water never reached levels greater than 0.07 mg L 1, compared to a maximum control the unplanted controls. Results indicated that no single concentration of 0.32 mg L 1 NH (Table 3). Leersia plant species was most effective at removing NH3, NO3 and PO4. oryzoides and Juncus effusus exhibited similar decreases in NH3 concentrations, with values typically around 0.10 mg L 1 NH less than measured in the controls up to the seventh hour of the experiment (Table 3). Statistically significant differences ( =0.05) in mean concentrations were noted between the control from three experiments, adjusted for 100% plant coverage.
Table 1. Mean percent coverage of mesocosm area and density of plants.
| Time | Control | Leersia | Juncus | Ludwigia |
|---|---|---|---|---|
| 0 | 0.01±0.02 | BDL | BDL | BDL |
| 1 | 0.12±0.09 | 0.02±0.03 | 0.01±0.02 | 0.01±0.02 |
| 2 | 0.14±0.14 | 0.03±0.04 | 0.04±0.06 | 0.01±0.04 |
| 3 | 0.18±0.09 | 0.08±0.06 | 0.13±0.08 | 0.03±0.03 |
| 4 | 0.30±0.11 | 0.16±0.04 | 0.17±0.09 | 0.06±0.07 |
| 5 | 0.28±0.12 | 0.20±0.07 | 0.18±0.08 | 0.07±0.06 |
| 6 | 0.32±0.14 | 0.24±0.09 | 0.21±0.09 | 0.04±0.05 |
| 7 | 0.15±0.12 | 0.24±0.05 | 0.24±0.12 | 0.04±0.05 |
| 8 | 0.20±0.10 | 0.15±0.10 | 0.16±0.06 | 0.01±0.02 |
| 9 | 0.22±0.00 | 0.18±0.07 | 0.07±0.04 | BDL |
*BDL = below detection limit of 0.001 mg L l.
Table 2. Water quality measurements in replicate mesocosms. Data recorded at 0700 prior to initiation of first experiment (July, 2002).
| Dissolved Oxygen (mg L1) | Temperature (°C) | Conductivity (pH) | Trough (µS cm) | |
|---|---|---|---|---|
| Juncus 1 | 1.57 | 23.2 | 6.0 | 71.2 |
| Juncus 2 | 1.45 | 23.1 | 6.0 | 55.9 |
| Juncus 3 | 1.53 | 23.1 | 6.2 | 57.9 |
| Leersia 1 | 0.82 | 23.3 | 5.9 | 49.6 |
| Leersia 2 | 2.08 | 23.3 | 6.1 | 46.1 |
| Leersia 3 | 1.72 | 23.2 | 6.0 | 62.0 |
| Ludwigia 1 | 4.03 | 23.2 | 5.8 | 23.4 |
| Ludwigia 2 | 3.50 | 23.2 | 5.7 | 29.2 |
| Ludwigia 3 | 3.51 | 23.2 | 6.2 | 26.2 |
| Control 1 | 7.04 | 23.2 | 7.0 | 54.7 |
| Control 2 | 6.43 | 23.2 | 6.9 | 55.7 |
| Reservoir | 7.60 | 23.2 | 6.9 | 124 |
Table 3. Mean ammonia concentrations in mg L l (±SD) from three experiments, adjusted for 100% plant coverage.
| Plant species | R1 | R2 | R3 | Mean | coverage shoots m2 |
|---|---|---|---|---|---|
| Ludwigia | 99 | 100 | 91 | 97 | 312 |
| Juncus | 28 | 44 | 85 | 52 | 2285 |
| Leersia | 98 | 100 | 99 | 99 | 820 |
R1: Replicate 1; R2: Replicate 2; R3: Replicate 3.
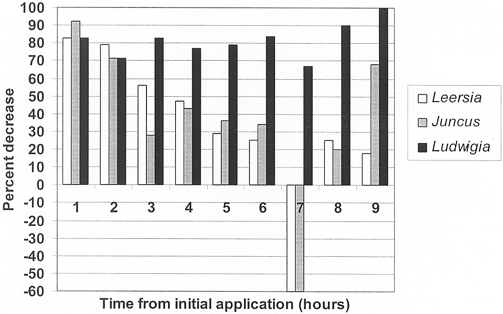
Figure 1. Ammonia concentration decrease (%) in outflow of planted mesocosms versus unvegetated controls.
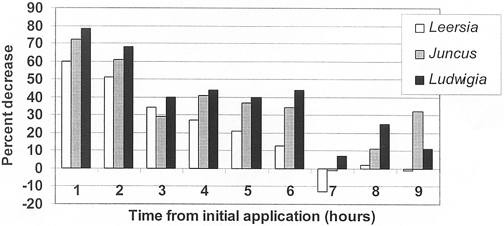
Figure 2. Nitrate concentration decrease (%) in outflow of planted mesocosms versus unvegetated controls.
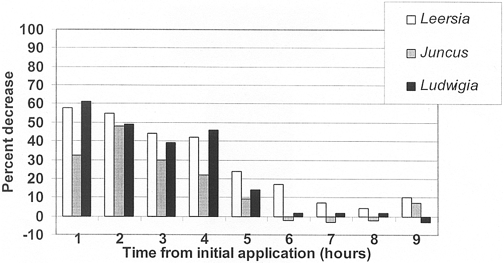
Figure 3. Total orthophosphate concentration decrease (%) in outflow of planted mesocosms versus unvegetated controls .
Table 4. Mean nitrate concentrations in mg L 1 (±SD) NH3 from three experiments, adjusted for 100% plant coverage.
| Time | Control | Leersia | Juncus | Ludwigia |
|---|---|---|---|---|
|
0 |
0.55±0.51 |
0.21±0.19 |
O.06±0.04 |
0.09±0.06 |
|
1 |
1.18±0.43 |
0.47±0.44 |
0.33±0.30 |
0.26±0.29 |
|
2 |
1.75±0.36 |
0.86±0.55 |
0.68±0.22 |
0.56±0.50 |
|
3 |
2.06±0.27 |
1.36±0.42 |
1.47±0.40 |
1.25±0.60 |
|
4 |
3.02±0.63 |
2.19±0.49 |
1.78±0.42 |
1.70±0.58 |
|
5 |
3.23±0.93 |
2.54±0.74 |
2.02±0.44 |
1.94±0.48 |
|
6 |
3.41±1.23 |
2.99±0.60 |
2.27±0.40 |
1.93±1.13 |
|
7 |
2.42±0.62 |
2.73±0.64 |
2.45±0.64 |
2.25±1.04 |
|
8 |
2.17±0.68 |
2.14±0.70 |
1.95±0.58 |
1.64±1.15 |
|
9 |
2.46±0.35 |
2.50±0.49 |
1.69±0.25 |
2.21±1.61 |
Table 5. Mean total orthophosphate concentrations in mg L 1 (±SD) from three experiments, adjusted for 100% plant coverage.
| Time | Control | Leersia | Juncus | Ludwigia |
|---|---|---|---|---|
|
0 |
2.11±1.17 |
0.83±0.33 |
0.99±0.18 |
0.66±0.28 |
|
1 |
3.18±0.82 |
1.35±0.49 |
2.16±0.70 |
1.23±0.65 |
|
2 |
4.42±0.70 |
2.00±0.42 |
2.58±0.88 |
2.24±1.05 |
|
3 |
5.08±0.39 |
2.87±0.32 |
3.56±0.40 |
3.09±1.09 |
|
4 |
5.97±1.03 |
3.49±0.64 |
4.67±0.94 |
3.25±0.78 |
|
5 |
5.55±0.74 |
4.20±0.73 |
5.04±1.38 |
4.79±0.53 |
|
6 |
5.61±0.25 |
4.68±0.73 |
5.75±0.51 |
5.52±0.56 |
|
7 |
5.46±0.24 |
5.10±0.62 |
5.60±0.78 |
5.35±0.14 |
|
8 |
4.52±0.18 |
4.35±0.61 |
4.61±0.52 |
4.45±0.28 |
|
9 |
4.49±0.24 |
4.05±0.49 |
4.16±0.21 |
4.64±0.54 |
Overall mean NH3 removal rates, based on a 4 h HRT, were 82±3% (Ludwigia peploides), 37±14% (Juncus effusus) and 34±14% (Leersia oryzoides) and Juncus effusus; control and Ludwigia peploides; Leersia oryzoides and Ludwigia peploides; and Juncus effusus and Ludwigia peploides.
Mean overall NO3 removal efficiencies were fairly low among the three species with Ludwigia peploides at 40±8%; Juncus effusus at 35±7%; and Leersia oryzoides at 22±8%. Ludwigia peploides and Juncus effusus were only slightly more effective than Leersia oryzoides (Figure 2; Table 4). There were no observed statistically significant differences in specific plant versus plant or in plant versus control mean concentrations of NO3. Likewise, no statistically significant differences were apparent between specific plant removal efficiencies.
Total PO4 removal efficiencies for the three species were greatest in Leersia oryzoides (29±7%) followed by Ludwigia peploides (24±8%) and Juncus effusus (16±6%) (Figure 3; Table 5). The only statistically significant difference occurred when comparing the mean concen- tration of the control mesocosm versus Leersia oryzoides. No significant plant specific differences in total PO4 removal were noted. Throughout the entire experiment, no one species was more efficient at removing both N and P constituents.
According to Surrency (1993) and Brix (1994), plants are beneficial for nutrient mitigation since they (1) are capable of some direct uptake in the water, (2) increase hydraulic retention time by decreasing water flow, (3) provide soil oxygenation and (4) provide surface area for microbial biofilms. Results of mesocosm studies indicated no single species was most effective at removing NH. The mechanism for this reduction in water column nutrient concen- tration is unknown. Most rooted emergent macro-phytes, such as those used in this study, are thought to take up nutrients primarily from sediments rather than directly from the water (Janse 1998). Silvan et al. (2004) reported that plant roots and storage organs of the cottonsedge, Eriophorum vaginatum, acted as long- term nutrient sinks. However, rooted submerged angiosperms take up nutrients from both water and sediment, and non-rooted submerged angiosperms and floating-leaved plants derive nutrients directly from water (Janse 1998). According to Sytsma (1989), Ludwigia peploides has the capability of absorbing nutrients from water through its extensive adventitious root system. In addition, algal species gain nutrients directly from water. In our study, pond water was pumped through all mesocosms for two days prior to the initiation of the study, so any algal species present should be similar in all tubs.
Howard-Williams (1981) conducted enrichment experiments on enclosures of a Potamogeton pectinatus community using the same initial forms of N and P (sodium nitrate and potassium phosphate dibasic) used in this study. Plant shoot density (approximately 1000 shoots m 2) for Potamogeton were similar to Ludwigia peploides densities (820 shoots m 2) in the current study. Howard-Williams (1981) found a rapid increase in growth of filamentous algae (Cladophara species) over a 9 week period and measured P absorbance to be eight times faster in Cladophara than Potamogeton. Although the study extended up to 23 weeks, they found nutrient concentrations in the water column were reduced rapidly, NO3 loads were reduced to control values within 5 h, and PO4 was removed within 1 d in even the highest treatments. Howard-Williams (1981) concluded that macrophyte beds such as this Potamogeton pectinatus community might be used in reduction of nutrients from agricultural runoff. Kuehn and Suberkropp (1998) reported a lack of significant nitrogen and phosphorus loss through leaching or mineralization from Juncus effusus litter. Results suggested both nutrients were either assimilated into microbial biomass, or they were in forms not readily leached by vegetation. Kao et al. (2003) determined that Juncus effusus had 87% nitrogen and 69% phosphorus remaining in litter after five months -more than Phalaris arundinacea (reed canary grass); Calamo- grostis canadensis (Michx.) Beauv. (blue joint grass); Sparganium americanum Nutt. (burreed) and Scirpus cyperinus (L.) Kunth (woolgrass).
Meuleman and Beltman (1993) reported on ditch systems used for water quality improvement in the Netherlands and found low removal of N, but 90-95% removal of P in an area with an abundance of brown moss Fontinalis antipyretica. Field and laboratory studies were conducted by Richardson and Marshall (1986) examining P movement in a minerotrophic peatland in central Michigan. In their study as nutrient availability increased, nutrient uptake and storage by the narrow- leaved sedge, Carex species, increased. Algal populations were an important temporary sink, removing P rapidly from the water column. They concluded that “soil adsorption and peat accumulation (i.e. phosphorus stored in organic matter) control long-term phosphate sequestration. But microorganism and small sediments control initial uptake rates, especially during periods of low nutrient concentrations and standing surface water” (Richardson and Marshall 1986).
Water temperature and pH are driving factors in the rate of denitrification in aqueous solutions. Other limiting factors include carbon availability and nitrate concentration (Trepel and Palmeri 2002). Sediment is a significant site for denitrification, due to a general abundance of organic carbon and the anaerobic environment (Bastviken et al. 2003). Much less known is the role of various aquatic plant species in the denitrification process (Bachand and Home 2000). Adsorption of nutrients onto sediment binding sites might account for some reduction in water column concentrations. All mesocosms were set up with the same sediments initially, but vegetated mesocosms developed an increase in sediment organic matter over time compared to the unvegetated controls. When exposed to nutrient enriched water, all plant treatments had total PO4 concentrations less than the control treatments through 5 h of the experiment (Table 5). The remaining 4 h of the experiment showed similar total PO4 concentrations in the controls and two of the plant treatments. Under anoxic conditions, dissolved inorganic P can be released from sediments (Howard- Williams 1981). Dissolved oxygen concentrations in planted mesocosms ranged from 4.03 to as low as 0.82 mg L 1 (Table 2) when measured at 0700, so it is likely that the majority of the sediments in these mesocosms were reduced. However, there does not appear to be any release of P from the sediments. Serrano et al. (1999) examined P in seasonal ponds after periods of drought and found that pond sediments were not a net source of P at the onset of the filling period. Instead they suggest P adsorption onto sediment particles. This has important implications for agricultural ditches that are periodically wet and dry.
Groffinan et al. (1992) suggested more nitrogen may be sequestered by plants than microbes during a growing season. Plants and microbes are capable of utilizing dissolved organic nutrients such as NH4, NO3 and PO4 (Dodds 2003). Bachand and Home (2000) reported the main mechanism for NO3 removal was due to bacterial denitrification, rather than plant uptake. However, the presence of plants is beneficial in other regards, since it increases the surface area for microbial biofilms (Eriksson and Weisner 1999). It is unknown if the reduced nutrient concentrations in the water column of plant treatments in this study are due to plant uptake, adsorption to the plant surface, or chemical processes such as precipitation, nitrification and denitrification. The mesocosms worked well for simulating a flow-through ditch environment and examining differences in mono cultures of hydrophytes. It is clear there are differences in nutrient removal between vegetated and unvegetated mesocosms and differences between species of emergent macrophytes. Additional work is needed to clarify the mechanism of nutrient reduction, as well as seasonal evaluations, and the possible effects of harvesting aboveground biomass.
Authors thank L. Arbuckle, L. Lee, T. Flemons and J. Walker for sample collection and analysis assistance. This manuscript is dedicated to the memory of the late J.D. Schreiber for providing improved experimental design suggestions and initial study support.
Ambrose, H.W. III and Ambrose, K.P. 1995. A Handbook of Biological Investigation. Fifth Edition. Hunter Text- books Inc., Winston-Salem, North Carolina, USA. 194 pages.
American Public Health Association (APHA). 1998. Standard Methods for the Examination of Water and Waste- water. 20th Edition. Washington, D.C., USA. 1113 pages.
Bachand, P.A.M. and Home, A.J. 2000. Denitrification in constructed free-water surface wetlands: II. Effects of vegetation and temperature. Ecological Engineering 14: 17-32.
Bastviken, S.K., Eriksson, P.G., Martins, I., Neto, J.M., Leonardson, L. and Tonderski, K. 2003. Potential nitrification and denitrification on different surfaces in a constructed wetland. Journal of Environmental Quality 32: 2414-2420.
Bengston, R.L., Carter, C.E., Fouss, J.L., Southwick, L.M. and Willis, G.H. 1995. Agricultural drainage and water quality in the Mississippi Delta. Journal of Irrigation and Drainage Engineering 121(4): 292-295.
Brix, H. 1994. Functions of macrophytes in constructed wetlands. Water Science and Technology 29: 71-78.
Carpenter, S.R., Caraco, N.R., Carrell, D.L., Howarth, R.W., Sharpley, A.N. and Smith, V.H. 1998. Non-point pollution of surface waters with phosphorus and nitrogen. Ecological Applications 8(3): 559-568.
Cooper, C.M., Moore, M.T., Bennett, E.R., Smith, S. Jr., Farris, J.L., Milam, C.D. and Shields, F.D. Jr. 2004. Innovative uses of vegetated drainage ditches for reducing agricultural runoff. Water Science and Technology 49(3): 117-123.
Dodds, W.K. 2003. Misuse of inorganic N and soluble reactive P concentrations to indicated nutrient status of surface waters. Journal of the North American Benthological Society 22(2): 171-181.
Donner, S. 2003. The impact of cropland cover on river nutrient levels in the Mississippi River Basin. Global Ecology and Biogeography 12: 341-355.
Eriksson, P.G. and Weisner, S.E.B. 1999. An experimental study on effects of submersed macrophytes on nitri- fication and denitrification in ammonium-rich aquatic systems. Limnology and Oceanography 44: 1993-1999. Goolsby, D.A., Battaglin, W.A., Aulenbach, B.T. and Hooper, RP. 2000. Nitrogen flux and sources in the Mississippi River Basin. Science of the Total Environment 248: 75-86.
Groffman, P.M., Gold, A.J. and Simmons, R.C. 1992. Nitrate dynamics in riparian forest: Microbial studies. Journal of Environmental Quality 21: 666-671.
Hammer, D.A. 1989. Constructed Wetlands for Wastewater Treatment: Municipal, Industrial and Agricultural. Lewis Publishers, Chelsea, MI, USA. 831 pages.
Howard-Williams, C. 1981. Studies on the ability of a Potamogeton pectinatus community to remove dissolved nitrogen and phosphorus compounds from lake water. Journal of Applied Ecology 18(2): 619-637.
Janse, J. 1998. A model of ditch vegetation in relation to eutrophication. Water Science and Technology 37(3): 139-149.
Kadlec, R.H. and Knight, R.L. 1996. Treatment Wetlands.
Lewis Publishers, Boca Raton, FL, USA.893 pages.
Kao, J.T., Titus, J.E. and Zhu, W.X. 2003. Differential nitrogen and phosphorus retention by five wetland plant species. Wetlands 23(4): 979-987.
Moore, M.T., Bennett, E.R., Cooper, C.M., Smith, S. Jr., Shields, F.D. Jr., Milam, C.D. and Farris, J.L. 2001.
Transport and fate of atrazine and lambda- cyhalothrin in an agricultural drainage ditch in the Mississippi Delta, USA. Agriculture Ecosystems and Environment 87: 309-314.
Moshiri, G.A. (Editor) 1993. Constructed Wetlands for Water Quality Improvement. Lewis Publishers, Boca Raton, FL, USA. 632 pages.
Richardson, C.J. and Marshall, P.E. 1986. Processes controlling movement, storage and export of phos- phorus in a fen peatland. Ecological Monographs 56(4): 279-302.
Serrano, L., Burgos, M.D., Diaz-Espejo, A. and Toja, J. 1999. Phosphorus inputs to wetlands following storm events after drought. Wetlands 19(2): 318-326.
Silvan, N., Vasander, H. and Laine, J. 2004. Vegetation is the main factor in nutrient retention in a constructed wet- land buffer. Plant and Soil 258: 179-187.
Surrency, D. 1993. Evaluation of aquatic plants for constructed wetlands. pages 349-357, In: Moshiri, G.A. (Editor) Constructed Wetlands for Water Quality Improvement. Lewis Publishers, Boca Raton, FL.
Sytsma, M. 1989. A study of growth, resource allocation and nutrient requirement of Myriophyllum aquaticum. Technical Progress Report for USGS Grant No. 14-08- 0001-G1626. University of California, Davis, CA. 32 pages.
Trepel, M. and Palmeri, L. 2002. Quantifying nitrogen retention in surface flow wetlands for environmental planning at the landscape-scale. Ecological Engineering 19(2): 127-140.